Bidang Material Untuk Energi dan Lingkungan
Bidang Kimia Material merupakan salah satu topik populer menurut American Chemical Society. Dalam rangka turut mengembangakan ilmu pengetahuan dan teknologi kimia namun tidak meninggalkan potensi sumber daya alam nasional Indonesia, kelompok penelitian material Program Studi Ilmu Kimia FMIPA UII menetapkan roadmap pengembangan penelitian material dalam skema Gambar 1.
Dari roadmap yang dikembangkan, sintesis material berbasis lempung alam telah diaplikasikan secara luas dalam bidang lingkungan dan industri kimia.
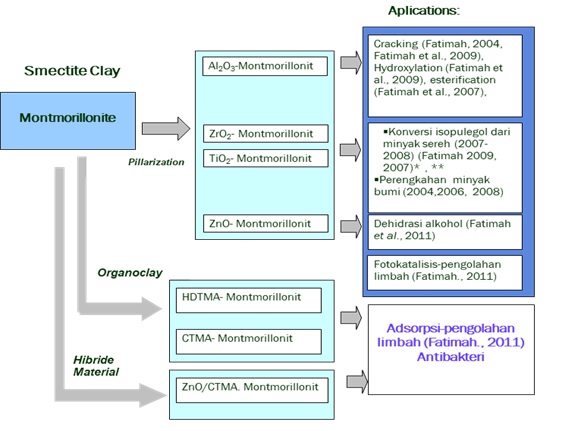
Gambar 1. Peta jalan (roadmap) penelitian pengembangan material montmorillonit (dipublikasikan Seminar Nasional Kimia VI
(Fatimah, 2011))
Rencana induk pengembangan penelitian dalam kurun waktu 5 (lima) tahun mendatang dapat disusun menurut skema berikut (Tabel 8). Lingkup penelitian material akan melibatkan pengembangan sumber daya mineral alam Indonesia maupun material sintetis yang berwawasan green chemistry. Material dalam lingkup tersebut meliputi:
a. Material dalam kelas zeolit dan lempung alam maupun sintetik
b. Material silika
c. Material karbida
d. Material berbasis selulosa alam, pati alam dan limbah industri pertanian dan karbon aktif
e. Material berbasis bahan tambang
f. Material semikonduktor
g. Material magnetik
h. Material berbasis kitin dan kitosan
i. Material polimer alam dan sintetik
Electrochemical Approaches to Humans and the Environment
Topic:
Application of electrochemistry for preparation of non enzyme sensor (electroanalytical), degradation of waste water (electrodegradation) and disinfection of water (electrodisinfection)
Sub Topic 1:
Preparation, characterization and application of glucose, uric acid and urea medical test using voltammetry cyclic composite electrode (non enzymatic sensor) fourth generation
Introductions
Diabetes mellitus is a worldwide public health problem. This metabolic disorder results from insulin deficiency and hyperglycemia and is reflected by blood glucose concentrations higher or lower than the normal range of 80-120 mg/dL (4.4-6.6 mM). The disease is one of the leading causes of death and disability in the world. The complications of battling diabetes are numerous, including higher risks of heart disease, kidney failure, or blindness. Such complications can be greatly reduced through stringent personal control of blood glucose. The diagnosis and management of diabetes mellitus thus requires a tight monitoring of blood glucose levels. Accordingly, millions of diabetics test their blood glucose levels daily, making glucose the most commonly tested analyte. Indeed, glucose biosensors account for about 85% of the entire biosensor market. Such huge market size makes diabetes a model disease for developing new biosensing concepts. The tremendous economic prospects associated with the management of diabetes along with the challenge of providing such reliable and tight glycemic control have thus led to a considerable amount of fascinating research and innovative detection strategies.1,2 Amperometric enzyme electrodes, based on glucose oxidase (GOx), have played a leading role in the move to simple easy-to-use blood sugar testing and are expected to play a similar role in the move toward continuous glucose monitoring (Wang 2008).
The history of glucose enzyme electrodes began in 1962 with the development of the first device by Clark and Lyons of the Cincinnati Children’s Hospital.3 Their first glucose enzyme electrode relied on a thin layer of GOx entrapped over an oxygen electrode via a semipermeable dialysis membrane. Measurements were made based on the monitoring of the oxygen consumed by the enzyme-catalyzed reaction. A negative potential was applied to the platinum cathode for a reductive detection of the oxygen consumption.
Uric acid is a metabolism product of purine in human body. The uric acid concentrations in urine and serum are in certain ranges for healthy persons (Yang et al. 2009). Therefore, the determination of UA concentration is helpful for the diagnosis of the some diseases such as gout, hyperuricaemia and Lesch-Nyhan syndrome. Consequently, the methods like chromatography, spectrophotometric and electroanalytical development for the detection of UA are usually employed. Electroanalytical methods, which are simple, sensitive, reliable, repeatability and low costs, are more convenient. Chemically modified electrodes have been used to determine the concentration of UA. These electrodes were used to be developed sensitive and selective methods for the detection. Polyaniline nano-networks on p-aminobenzene sulfonic acid functionalized glassy carbon electrode were used for the simultaneous determination of ascorbic acid and uric acid. Poly(4-aminobenzene sulfonic acid) modified glassy carbon electrode was used determination of phenylephrine and chlorprothixene. In addition, this electrode was used also for selective determination of hydroquinone in the presence of catechol and resorcinol. However, the determination of UA at the p-ABSA modified glassy carbon electrode has not yet been reported up to date.
The determination of urea is essential for a number of applications, including clinical diagnosis, food processing, agricultural process and environmental protection. Because of very fast and high expansion of industrial and farming activity, the presence of urea in water is increasing. Also, high quantities of urea are present in municipal wastewaters because about 80% the nitrogen in fresh urine is bound in urea. Several analytical procedures have been developed for the analysis of urea in aqueous samples. Most of these investigations on the determination of urea have been based on measuring the changes of ammonia enzymatically released during the hydrolysis of urea. There are several analytical procedure based on the optical methods and electrochemical techniques. In general, the assay of urea by electrochemical techniques means the use of enzyme-modified electrodes by potentiometric and amperometric methods. Though, a non-enzimatic amperometric detection of urea in aqueous solution at poly(Ni-cyclam) film-modified glassy carbon electrode (De Melo et al. 2002; Singh et al. 2008).
First-generation glucose biosensors rely on the use of the natural oxygen cosubstrate and generation and detection of hydrogen peroxide. The biocatalytic reaction involves reduction of the flavin group (FAD) in the enzyme by reaction with glucose to give the reduced form of the enzyme (FADH2) (Wang, 2008).
Tool test glucose, uric acid and urea are sold today use an enzyme known as biosensors. The technique used is amperometric enzyme electrodes. Products sold under the name Easy Touch Blood Glucose, cholesterol, uric acid meter (Easy Touch GCU) at a price of $ 57.99 or approximately Rp. 800.000, -. This technique has a short durability, because the enzyme is not stable. Therefore, research of preparation, characterization and application of glucose, uric acid and urea medical test using voltammetry cyclic composite electrode (non enzymatic sensor) fourth generation is a very importance. Disclaimer of this tool test:
1. The self-testing Easy Touch GCU Blood Glucose/Cholesterol/Uric Acid Multifuction Monitoring System is designed for in vitro diagnostic use only (external use only).
2. Do not change your medical plan without doctors approval.
3. The Easy Touch GCU system should not be used for the diagnosis of diabetes, hypercholesterolemia and hyperuricemia or for the newborns.
4. Blood specimens containing ascorbic acid (Vitamin C) greater than 150mg/dL, Amiloride greater than 20 mg/dL, acetaminophen greater than 8mg/dL, L-Dope greater than 20mg/dl, Dopamine greater than 20 mg/dL, Methyl-Dope greater than 4 mg/dL, Galactose greater than 400 mg/dL, uric acid greater than 10.5 mg/dL and Xylose greater than 50mg/dL may give inaccurate glucose results.
5. Blood specimens containing ascorbic acid (Vitamin C) greater than 5mg/dL, acetaminophen greater than 15mg/dL, L-Dopa greater than 1.25 mg/dL, Dopamine greater than 3 mg/dL, Methyl-Dopa greater than 5 mg/dL, Glibenclimide greater than 10 mg/dL, creatine greater than 20 mg/dL and Billirubin greater than 20 mg/dL may give inaccurate cholesterol results.
6. Blood specimens containing ascorbic acid (Vitamin C) greater than 10 mg/dL, acertaminophen greater than 2 mg/dL, L-Dopa greater than 20 mg/dL, Dopamine greater than 5 mg/dL, Methyl-Dopa greater than 0.3 mg/dL, Glibenclimide greater than 10 mg/dL, creatine greater than 30 mg/dL, Bilirubin greater than 40 mg/dL and ketoprofen greater than 300 mg/dL may give inaccurate uric acid results.
Zeolite-modified electrodes (ZMEs) have been drawing attention as chemically modified electrodes (CMEs) because of synergistic combination of zeolite features with electrochemical interfaces. ZME can be exploited as electrochemical sensors in relation with zeolite’s properties, e.g., ionexchange capacity, molecular selectivity, and catalyst-assisted reactivity. Moreover, metal ion-doped zeolites allow exploitation of ion-exchange capacity of zeolite for the development of electrochemical sensors for the sensing non-electroactive inorganic or organic species. Also, the zeolite supported electrocatalyst can be exploited to improve the performance of analytical sensing device. For the electrochemical detemination of urea, the clinoptilolite has been exploited for the preparation of biosensors by covalent binding of enzymes to the surface of an ion-sensitive membrane made up of a zeolite-polymer matrix. In this situation, the electrochemical strategy was based on the clinoptilolite affinity for ammonium to determine urea using urease. The main disadvantages of the ammonium sensor were its long response time and the limited stability of the urease layer (Soy 2011; Teles and Fonseca 2008).
Chitosan functionalized graphene was synthesized by a together-blending and in situ chemical reduction method (Suginta et al. 2010; Srivastava et al. 2014). UV-vis, FT-IR, Raman and SEM techniques were used to study the molecular structure and morphology of the resulting composite. The chitosan-graphene modified glassy carbon electrode showed high electrocatalytic activity towards oxidations of ascorbic acid (AA), dopamine (DA) and uric acid (UA). By using the differential pulse voltammetry (DPV) as the analytical technique the calibration curves for AA, DA and UA, determined simultaneously, were found linear with a concentration range of 50–1200 μM, 1.0–24 μM and 2.0–45 μM, respectively (Han et al. 2010). Orata et. al. (2014) the surface of the working electrode was modified using electronically conducting polymer polyanailine and a clay montmorillonite bentonite. The same analysis was repeated but this time using bentonite modified electrode. We obtained a broad poorly defined cyclic voltammogram (Mousty 2004; Takahashi and Anzai 2013).
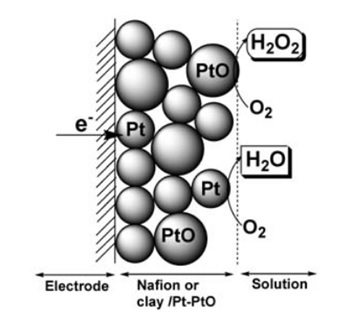
Figure 1. Schematic representation of process at nafion or clay/Pt-PtO electrode
In the present work, the preparation, characterization and application of glucose, uric acid and urea medical test using voltammetry cyclic composite electrode (non enzymatic sensor) fourth generation. Composite electrode was preparation using chitosan, zeolite and clay was used for binding electrode. The low cost, high stability, simplicity and reproducibility and easy fabrication this product could be an alternative method in the detection of glucose, uric acid and urea in routine clinical and pharmaceutical analysis.
Parts of the Research Results
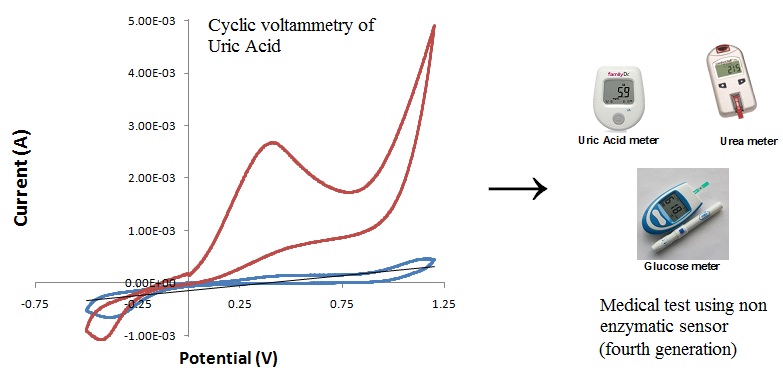
Figure 2. Preparation of medical test using non enzymatic sensor fourth generation
The Road Map Research
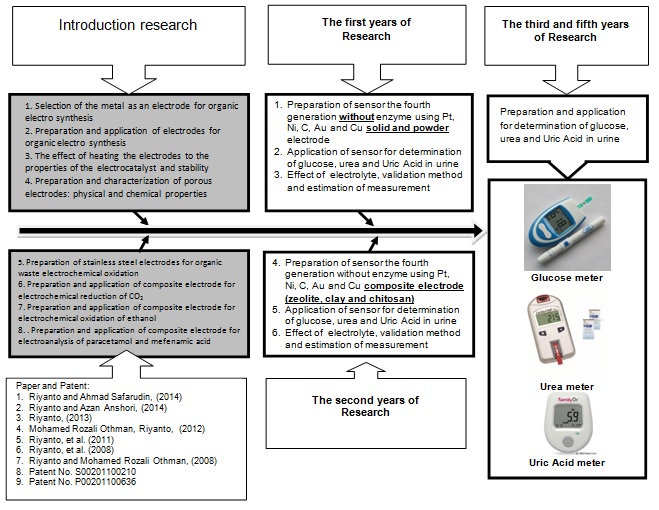
Figure 3. The Road Map Research topic 1
Sub Topic 2
Preparation, Characterization and Application of Carbon-Modified Electrode for Electrochemical Degradation of Waste Water Batik, Textile and Laundry
Introduction
The presence of dyes in textile effluents is also common and highly visible. Depending on the type of dyestuff used, the colour of the wastewater can change from day to day, or even several times a day because the dyestuff used in the dyeing process changes frequently due to customer’s requirements. The large pH swing in the textile wastewater (can change from 2 to over 12) is another strong negative point. This variation is primarily caused by different kinds of dyestuff used in the dyeing process.
Textile industry causes considerable higher impacts to water pollution by discharging their effluents into various receiving bodies includes ponds, rivers and other public sewer. Major pollutant load from the textile industries are from the several of their wet-processing operations like scouring, bleaching, mercerizing and dyeing. Among these various processes, dyeing process normally uses large amount of water for dyeing, fixing and washing processes. Thus, textile wastewater possess a high COD concentration, large amount of suspended solids, broadly fluctuating pH, strong color, high temperature and low biodegradability caused by varying contaminates within water environment.
Nowadays, there is increasing interest in development of innovative for treatments of textile wastewater that contain toxic and non-biodegradable organic pollutants which are traditional method cannot completely oxidize. Based on the previously research, biological treatment of textile wastewater showed low degradation efficiency because of the presence of biologically inert high molecular weight dyestuff. Physical adsorption is effective for removal of non-biodegradable pollutants, but it is quite expensive and difficult for regenerating the adsorbents. Due to the large complexity of the composition in textile wastewater, most of these traditional methods are becoming inadequate. In recent years, there has been increasing interest in the use of electrochemical technique for the treatment of textile wastewater. In electrooxidation, the main reagent that is used here is electron (clean reagent).
Water pollution with detergents, is of great importance to satisfy the increasing demands for water for various uses. These detergents compounds do not decompose or degrade in aquatic systems. These detergents are very harmfully and toxic. The accumulation of some detergents in waste water represents a serious environmental problem. The removal of detergents from aqueous solutions is very important from the environmental point of view (El-Said 2004).
Detergents are substances or preparations containing soaps or other surfactants intended for water based laundry or dishwashing processes. Detergents may be used in any form (liquid, powder, paste, bar, cake, molded piece, shape, etc.), widely for household laundry products, domestic and industrial cleaners, cosmetic products, and industrial purposes. Surfactants are organic substances, used in detergents, intentionally added to achieve cleaning, rinsing and/or fabric softening due to its surface-active properties (Bruns and Jelen 2009). They consist of one or more hydrophilic and hydrophobic groups of such nature and size that they are capable of forming micelles Surfactants belong to a group of chemicals of high environmental relevance due to their large production volumes. They are mainly discharged into the environment by the wastewater pathway, either after treatment in a wastewater treatment plant or directly where no treatment system is available. Environmental compartments which may be influenced by surfactants are the freshwater environment (water body and sediment), the soil if surfactant-loaded sewage sludge is added, and the marine environment (Bruns and Jelen 2009). Surfactants are widely used for domestic and industrial purposes, primarily as detergents in cleaning applications.
Surfactants removal operations involve processes such as chemical and electrochemical oxidation (Lissens, et al., 2003; Mozia, et al., 2005), membrane technology (Sirieix-Plénet, et al., 2003; Kowalska, et al., 2004; Fernández , et al., 2005), chemical precipitation (Talens-Alesson, et al., 2002), photocatalytic degradation (Ohtaki, et al., 2000; Zhang, et al., 2003), adsorption (Ogita, et al., 2000; Lin, et al., 2002; Adak, et al., 2005) and various biological methods (Dhouib, et al., 2003; Chen, et al.,2005). Among the currently employed chemical unit processes in wastewater treatment, coagulation-flocculation has received considerable attention for yielding high pollutant removal efficiency. This process can be directly applied to wastewaters without being affected by the toxicity in the wastewater and can constitute a simple, selective and economically acceptable alternative.
Electrochemical technologies such as electrolysis have been successfully employed for the treatment of many wastewaters on an industrial scale, for example, oil and grease (O&G) containing wastewaters. The electrochemical technologies have reached such a state that they are not only comparable with other technologies in terms of cost but also are more efficient and more compact (Dae et al. 2013). The electrochemical oxidation of detergent to CO2 occurs without chemical agent and with a significant rate the potential region of oxygen evolution. It is commonly assumed that electrogenerated hydroxyl radicals are very active in the degradation of organic molecules. This species is the most powerful oxidant in water.
This paper reports a study of the electrocoagulation of detergent wastewater. Electrochemical degradation of organic pollutants, presence in the wastewater needs specific electrodes (Aboulhasan et al. 2006). Electro coagulation experiments on detergent were carried out with aluminium wire netting electrode. Electrocoagulation involves the in situ generation of coagulants by dissolving electrically either aluminum or iron ions from aluminum or iron electrodes, respectively. The metal ions generation takes place at the anode, hydrogen gas is released from the cathode. The hydrogen gas would also help to float the flocculated particles out of the water. This process sometimes is called electroflocculation (Songsak, 2006).
Parts of the Research Results
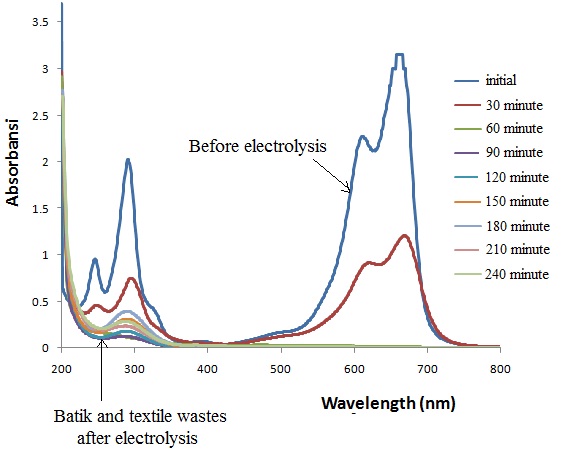
Figure 4. Electrochemical degradation of batik, laundry and textile waste
The Road Map Research
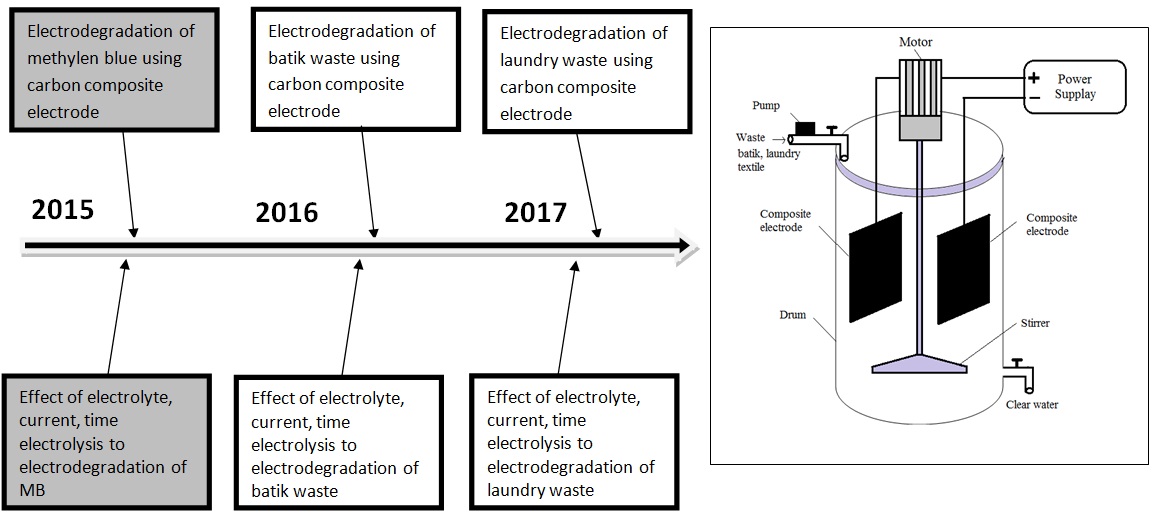
Figure 5. The road map research topic 2
Sub Topic 3
Electrochemical Disinfection of Bacteria in Drinking Water Using Carbon Composite Electrode
Introduction
In general, the microbial cell can be damaged as a consequence of adverse conditions, such as osmotic pressure difference, electric field, desiccation, heating, chilling, chemicals, oxidants or ultraviolet radiation. Based on such mechanisms, many of drinking water treatment systems have been developed and tested, using osmosis, flocculation, UV light irradiation (Kalisvaart, 2004), ultrasonic irradiation, ozone, hydrogen peroxide, photolysis, homogeneous and heterogeneous photocatalysis, radiolysis, biocidal agents, high-voltage pulses (Lubicki and Jayaram, 1997), electrohydraulic discharges, electrolysis, and combined techniques. However, recent research has shown that some treatment methods, such as chlorination and UV radiation with low pressure lamps, are not fully efficient, as some pathogens are capable of “reactivation” or “recovery”; these include Escherichia coli,Campylobacter jejuni, enteric viruses such as rotavirus, calicivirus, and astrovirus and the parasites Cryptosporidium parvum, Giardialamblia and microsporidia (Szewzyk et al., 2000).
Moreover, the chlorination of drinking water may affect its taste and odor and generate hazardous oxidation by-products during the treatment, mainly chloramines and trihalomethanes (THM). These chemicals are established mutagens and carcinogens, arising from reactions between chlorine and amines or chlorine and organic matter, respectively, and mainly affect the gastrointestinal tract (Kimbrough and Suffet, 2002). Also, long-time exposure to chlorite causes anemia and a significant increase in methaemoglobinemia (Condie, 1990).
Water disinfection (Wadis) is one of the main stages in water and waste water treatment that ensures the killing of all hazardous microorganisms that can harm a human being. The conventional methods for water disinfection include chemical methods such as chlorine treatment and ozone treatment, and physical methods such as ultraviolet treatment. Each method has its own disadvantages such as DBP’s (Disinfection by Product) formation, storage, expensive maintenance and high energy consumption. Wadis technology is a non reagents ecological method based on electrical pulse discharge in water. A high electrical voltage discharge within water causes a strong shock wave, high light radiation including UV, high magnetic and electrical fields, ionization and polarization of molecules, cavitations and very high temperatures at the discharge canal. All these act as disinfection agents, ensuring a high performance system. The company’s unique system is effective against a wide range of microorganisms, effective in various applications and achieves a total bacteria killing while using low energy consumption.
Pollution of natural water, which is primary resource for survival of mankind, is evidently gaining concerning scale. Vulnerability of open springs, waterfowls, natural lakes, artificial accumulations as well as natural wells (open and underground waters) is direct consequence of ever increasing pollution of environment. Besides unacceptably increased concentrations of physico-chemical constituents in raw water, new emerging problem is bacterial, i.e. microbiological water pollution. Within water treatment, process of disinfection has highly important role.
Electrochemical water disinfection is a rarely used but convenient and highly efficient way to produce germ-free water. The technique works without the addition of chemical compounds to the water to be treated, but is nevertheless based on the biocidal action of various chemical substances. Electrodes with platinum group metals (pgms) or their oxides as active coatings are generally the best suited to electrochemical water disinfection. Electrochemical water disinfection can be defined as the eradication of microorganisms by using an electric current passed through the water under treatment by means of suitable electrodes. At the phase boundary between the electrodes and the water, the electric current leads to the electrochemical production of disinfecting species from the water itself (for example, ozone), or from species dissolved in the water (for example, chloride is oxidized to free chlorine).
Until recently none have been successful, at least not for long-term practical use. Different terms are or have been in use to describe this type of water treatment process or the water produced by this process, such as ‘electrolytic disinfection’, ‘electrochemical water’ and ‘electrochemically activated water ‘among others. There are three reasons why electrochemical water disinfection has arrived at technical maturity only recently, rather than earlier in the (possibly) 2000 years since its discovery: (a) sufficiently stable and efficient electrode materials for electrochemical water disinfection have been developed and optimized only in the last forty years. These are titanium electrodes with mixed oxide coatings based on iridium and/or ruthenium oxide, and doped diamond electrodes. (b)The functional interrelationships between chloride concentration in the water, current, current density, electrode material, water quality, electrochemical production of free chlorine and disinfecting action have been investigated in detail only recently. (c) Development work on electrochemical water disinfection has often been undertaken by amateurs in both electrochemistry and water chemistry, and this remains somewhat true today. Only a few electrochemists have been interested in this topic, mostly only for a short period in their career. This has resulted in mistakes in device dimensioning and in unscientific explanations of the mechanism of the process.
Electrochemical treatment is an efficient method for the removal of pathogens. The method was used for disinfection in food processing from neutral electrolyzed water containing active chlorine (Deza et al., 2003), to promote the inactivation of Legionella in circulating water with 0.1% NaCl used in cultivation of germinated brown rice (Feng et al., 2004) and in medical applications (Tsuji et al., 2000). Diao et al. (2004) tested E. coli disinfection by various treatments, including chlorination, ozonation, Fenton reaction and electrochemical disinfection using 500 mg L 1 of NaCl as electrolyte. Scanning electron microscopy analysis suggested that the electrochemical treatment had a greater effect than the other disinfection processes examined.
Various electrode materials have been tested for electrochemical water disinfection, with a focus on the applied cell potentials and killing mechanisms. Model water disinfection with electrolysis using TinO2n-1 containing ceramic electrodes has been done by Reimanis (Reimanis et al. 2011). Electrochemical disinfection of bacteria in drinking water using activated carbon fibers (Matsunaga et al. 1994).
The Road Map Research
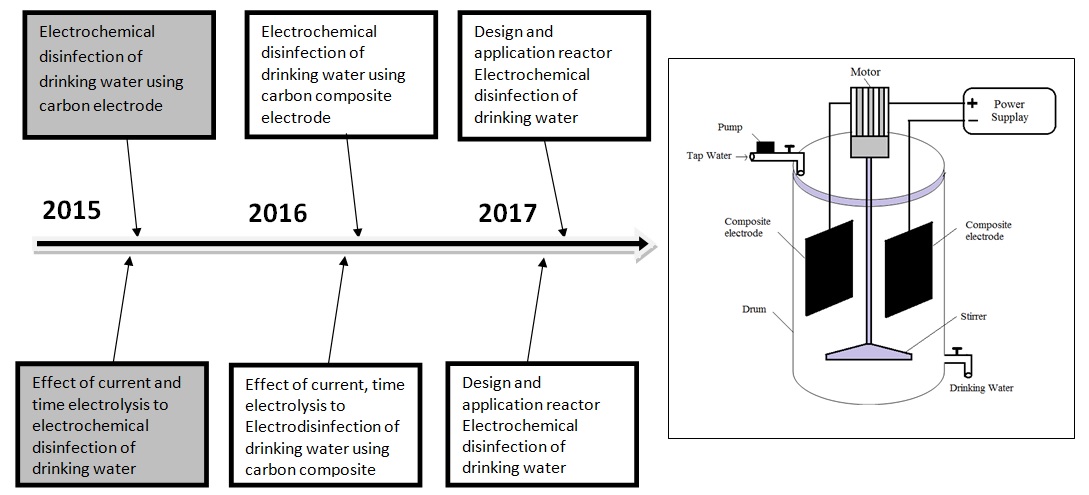
Figure 6. The road map research topic 3
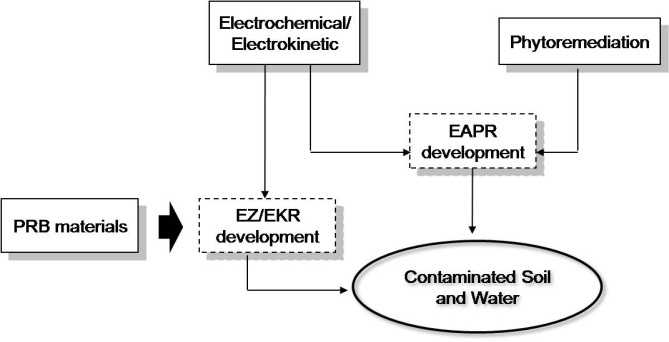
Kelompok riset remidiasi lingkungan — Pengembangan metode remediasi tanah dan air tercemar dengan proses EAPR, EZ/EK dan elektrokoagulasi
Tujuan tema penelitian ini adalah untuk melakukan evaluasi terhadap gabungan proses EAPR (electro-assisted pytoremediation) dan proses EZ/EK (entrapping zone/electrokinetic) serta proses elektrokoagulasi untuk remediasi tanah dan air yang tercemar oleh logam berat. Gambar 1 menunjukkan diagram skematik penelitian yang sedang dikembangkan di kelompok riset remediasi tanah dan air.
Fitoremediasi merupakan teknik pemanfaatan tumbuhan untuk mengurangi dan menurunkan ketersediaan kontaminan dalam tanah atau air. Beberapa keunggulan dari metode fitoremediasi ini antara lain tanaman memiliki kemampuan untuk mengurangi konsentrasi logam berat. Logam berat tersebut akan terakumulasi pada akar kemudian mengalami translokasi ke bagian batang dan daun tanaman. Akan tetapi proses fitoremediasi juga memiliki beberapa kelemahan diantaranya akar tanaman yang pendek serta pertumbuhan biomassa yang lambat. Selain itu, pada fitoremediasi proses pembersihan berlangsung relatif lama. Beberapa kelemahan yang dimiliki oleh proses fitoremediasi tersebut kemudian diatasi melalui gabungan proses fitoremediasi dengan elektrokimia yang selanjutnya dikenalkan dengan istilah electro-assisted phytoremediation (EAPR). Dalam sistem EAPR, elektroda yang digunakan akan berfungsi untuk mobilisasi ion logam melalui proses elektromigrasi sehingga ion logam akan terdorong dan terakumulasi pada daerah akar tanaman yang selanjutnya diikuti dengan proses absorpsi oleh akar tanaman. Keunggulan metode EAPR adalah di mungkinkannya untuk menggunakan tanaman yang memiliki akar pendek sehingga akan mengatasi kekurangan yang tedapat pada proses fitoremediasi. Gambar 2 menunjukkan aplikasi proses EAPR untuk remidiasi air tercemar logam Pb.
Gambar 1. Skema penelitian di kelompok riset remediasi air dan tanah

Gambar 2. Reaktor sistim EAPR (A) dan titk sampling air pada reaktor (B) dan katoda berbentuk pot (C)
Kelompok riset energy baru dan terbarukan – Aplikasi proses elektrolisis untuk produksi biodiesel dengan katalis asam dan basa
Bahan bakar minyak adalah sumber energi dengan konsumsi terbesar bila dibandingkan dengan sumber energi lain sehingga saat ini dunia menghadapi krisis bahan bakar minyak. Keterbatasan energi yang berasal dari minyak bumi telah menuntut Indonesia untuk mencari sumber energi lain yang ketersediaannya dapat diperbaharui. Biodiesel dipandang sebagai bahan bakar alternatif masa depan yang dapat digunakan sebagai pengganti minyak bumi. Keunggulan biodiesel sebagai bahan bakar pengganti solar adalah sifatnya ramah lingkungan, non-toxic, renewable, dan biodegradable serta bahan baku yang berlimpah berasal dari minyak nabati dan lemak hewan. Rencana penelitian lanjutan akan dikembangkan pada pembuatan biodiesel dengan gabungan metode modifikasi proses elektrolisis dan katalis heterogen kitosan yang termodifikasi dalam fase sol-gel (hydrogel, xerogel, cryogel dan aerogel). Keunggulan lain dari metode ini adalah kandungan air yang terkandung di dalam minyak jelantah yang biasanya bermasalah terhadap kualitas biodiesel, akan termanfaatkan pada proses elektrolisis sehingga menghasilkan biodiesel dengan kandungan air rendah (< 1%) dengan hasil konversi tinggi (≥ 97%). Penelitian sebelumnya telah menunjukkan keberhasilan penggunaan gabungan proses elektrolisis dengan kitosan sebagai katalis basa heterogen pada proses produksi biodiesel dari minyak jelantah dengan hasil konversi rendah sebesar 59.1 % (Putra dkk. (2014)) seperti yang ditunjukkan pada Gambar 3. Perbaikan pada penelitian ini dilakukan dengan memodifikasi katalis kitosan dalam bentuk fasa gel. Katalis tersebut akan disalutkan pada permukaan elektroda grafit, sehingga diharapkan paduan elektroda grafit dan kitosan gel akan dapat meningkakan suasana basa pada proses transesterifikasi minyak jelantah menjadi biodiesel. Gabungan kedua metode tersebut kemudian akan digunakan untuk mengembangkan proto-type produksi biodiesel dengan menggunakan elektroda tersalut kitosan gel pada proses elektrolisis seperti yang ditunjukkan pada Gambar 4.
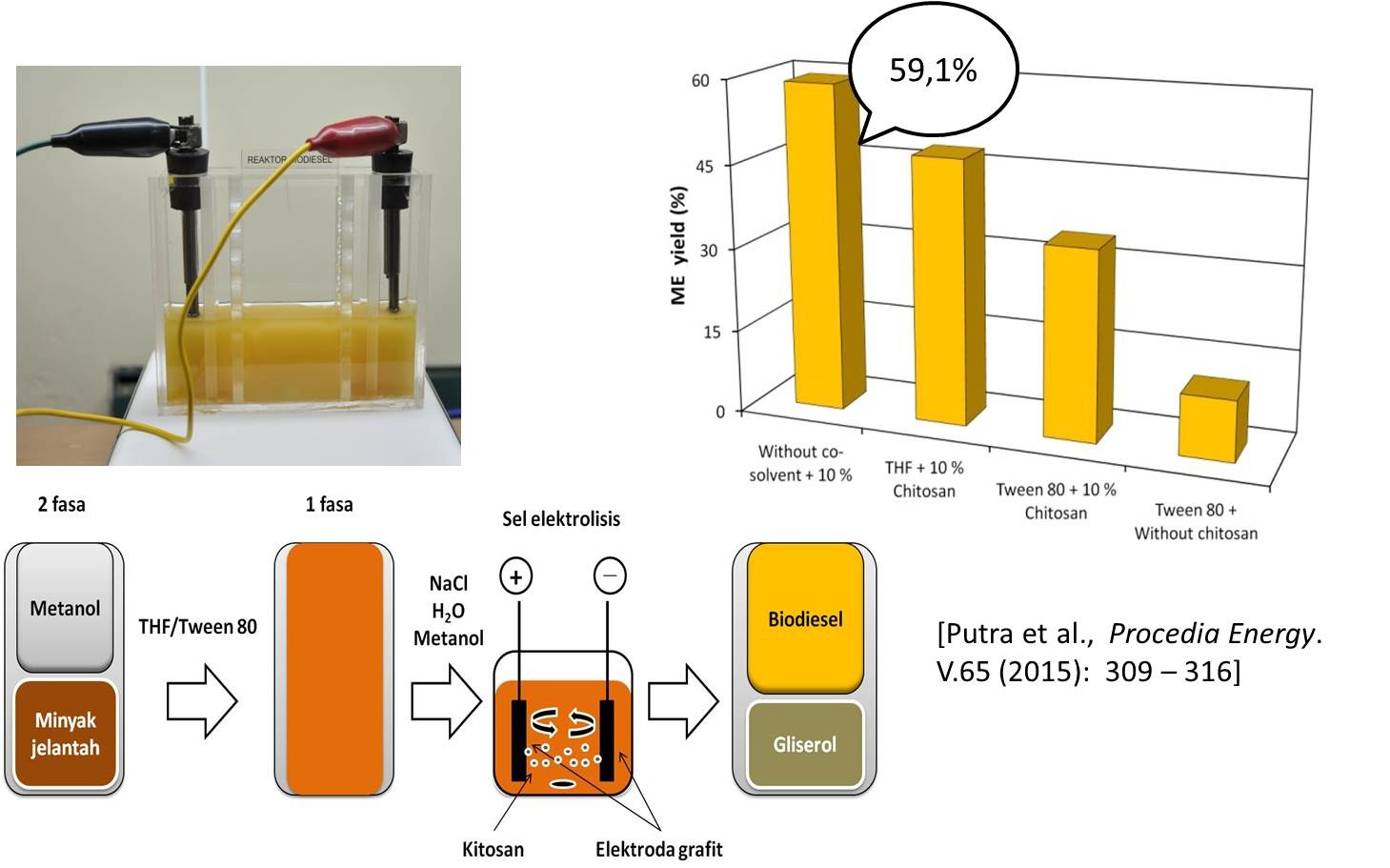
Gambar 3. Produksi biodiesel dengan proses elektrolisis dan katalis kitosan
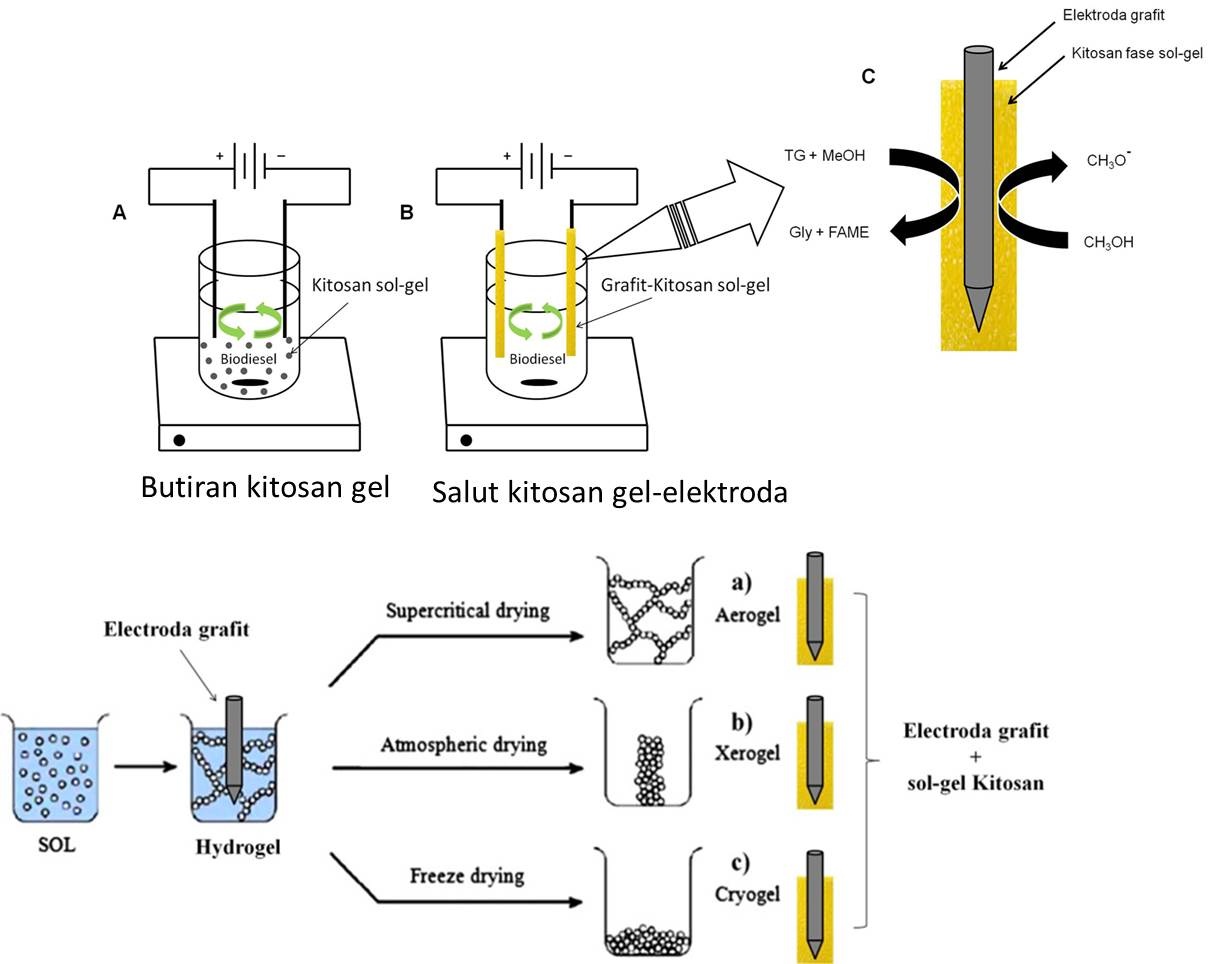
Gambar 4. Skema produksi biodiesel dengan proses elektrolisis menggunakan elektoda grafit yang tersalut kitosan gel





