Prof. Dr. Is Fatimah, S.Si., M.Si
Date of birth:
Bantul, 29 March 1975
Address:
Bulusan RT.03/RW.39, Sardonoharjo,
Ngaglik, Sleman DI Yogyakarta
Email: [email protected]

Date of birth:
Bantul, 29 March 1975
Address:
Bulusan RT.03/RW.39, Sardonoharjo,
Ngaglik, Sleman DI Yogyakarta
Email: [email protected]
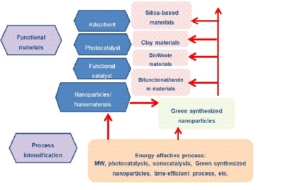
Functional Material for Energy and Environment
Environmental and energy crisis problems become important issues in last decade, and it will get more intensive attention in the next years. Due to this background, functional material development for the energy and environmental applications will be interesting and challenging topics for research activities. With the goal of green chemistry for sustainable development, the focus of research will be on the low cost technique for environmental protection including heterogeneous catalysis, photocatalysts, adsorption and nanomaterial for energy and environmental issues. Here are the detail of topics:
Heterogeneou catalysis for energy issues
In the renewable energy perspective, low cost and economic conversion of biodiesel get more attention for adaptive and applicability in small industry scale. The replacement of NaOH or KOH as homogeneous base catalysts with many heterogeneous solid acid and base catalysts have been investigated for a perspective of green chemistry, particularly for sustainable and reusable material aspects. The best way to enhance the economic value of the catalytic process for biodiesel production is to use agricultural waste as catalytic materials. Previous works reported potency of agricultural waste ash as precursor or support of catalyst for biodiesel conversion. In next years, the development of low cost catalayst for biodiesel conversion will be derived from biogenic silica-based materials, waste material from animals (CaO), and clay-based material.
Heterogeneou catalysis and intensification for sustainable reaction
Another chance for greener reaction enhancement will be attempted by developing heterogeneous catalyst and intensification using simpler and energy efficient process such as microwave (MW) irradiation, sonocatalytic and tandem reactions.
Photocatalysis
Dyes removal from wastewater is the main problem encountered in textile industry, instead of fate organic contaminated water. Color is a visible pollutant containing dyes which are organic compounds and therefore, it is required that the organic contamination in effluents be treated before they are discharged into aquatic environment. In this field of research a novel technology to remove the contaminants by advance oxidation process (AOPs) by using Photo-Fenton like process was widely developed. Photocatalysis utilizing titanium dioxide (TiO2) has gained great interest due to not only its high photocatalytic activity, chemical stability, and low cost but also from its band gap energy (anatase, 3.2 eV) allowable for UV excitation can be obtained from solar spectrum. Furthermore, in photo-Fenton like process, combination of photoactive material will be alternative process that can be operated in effective, easy to control and also cost efficient condition. Some effort concerned with modification of photo-Fenton catalyst with some inorganic supports were reported, and will be the focus of the research group. A wide range of solid materials, such as transition metal exchanged-zeolites and pillared clays have been reported to be active for oxidative degradation of organic compounds involved dye compounds through the photo-Fenton-like reaction.
Adsorption
Adsorption is one of the attractive methods for the removal of metal ions or organic contaminant in water environment in the terms of simplicity, suitability for low concentration, availability of different low-cost adsorbents and easy in application. Along with the need of low adsorbent, many inorganic and organic materials were developed from waste. Instead of carbon-based materials such as biochar, biosorbent and activated carbon that could be derived from agricultural waste, calcium-based materials were also widely studied.
Pengalaman Riset:
1. Fotodegradasi Zat Warna Rhodamin B menggunakan katalis Bentonit Terpilar Oksida Zr 2004/Lembaga penelitian UII.
2. Sintesis Lempung Terpilar Oksida Ti dan Aplikasinya sebagai Katalis pada Fotodegradasi Limbah Cair Industri Tekstil 2004/ KOPERTIS Wilayah V.
3. Penerapan Metode Adsorpsi-Fotodegradasi Limbah Cair Industri Tekstil menggunakan TiO2/Zeolit Bogasari Nugraha VII 2004/ PT. Indofood Sukses Makmur Bogasari Fluor Mills.
4. Sintesis Material Zeolitik Terembani TiO2 dari Abu Layang dan Aplikasinya pada Desinfeksi bakteri E.Coli Penelitian Dosen Muda Dirjen DIKTI 2006.
5. Sintesis dan Karakterisasi ZrO2-Montmorillonit sebagai Katalis Cracking Fraksi Berat Minyak Bumi Penelitian Dosen Muda Dirjen DIKTI 2007.
6. Sintesis ZrO2-Montmorillonit sebagai Fotokatalis pada Degradasi Metilena Biru 2006/ KOPERTIS Wilayah V .
7. Modifikasi Zeolit Alam dengan TiO2 dan Aplikasinya Sebagai Bahan Fotokatalis Untuk Degradasi Zat Warna Industri Tekstil RUT/Kementrian Riset dan Teknologi RI/2005-2006.
8. Pengembangan Katalis Selective Catalytic Reduction Untuk Mengurangi Emisi Gas Buang Kendaraan Berbahan Bakar Bensin Berbasis Pengemban Zeolit Alam Program Insentif Riset Terapan/2007.
9. Sintesis Nanokomposit ZrO2-montmorillonit dan TiO2-montmorillonit dan Uji Aktivitasnya Pada Konversi Isopulegol dari Minyak Daun Sereh Hibah Bersaing XVI/ Dirjen DIKTI 2007-2008.
10. Pemanfaatan Pt-Zeolit Alam Sebagai Katalis Konverter Gas Buang Kendaraan BermotorHibah Bersaing XVII/ Dirjen DIKTI 2008.
11. Sintesis TiO2 teremban pada Al2O3-saponit UBER-HKI Dirjen DIKTI 2008
12. 2010 Kajian Kinetika Dehidrasi Metanol menggunakan katalis ZnO/Montmorillonit Hibah Penelitian Fundamental DP2M-DIKTI
13. 2011 Composite of TiO2-montmorillonite –Extract of Garcinia Mangostana as component in Dye Sensitized Solar Cell L’oreal-UNESCO for Women in Science 2011
14. 2013 Membran nanokeramik Berbasis TiO2-Clay sebagai Desinfeksi Air Minum Insentif Riset Nasional 2013
15. 2014 Membran nanokeramik Berbasis TiO2-Clay sebagai Desinfeksi Air Minum Insentif Riset Nasional 2013
16. 2013 Konversi Hijau Sitronellal menggunakan katalis ZrO2-Montmorillonit Tersulfasi Hibah Unggulan PT DP2M-DIKTI
17. 2014 Konversi Hijau Sitronellal menggunakan katalis ZrO2-Montmorillonit Tersulfasi Hibah Unggulan PT DP2M-DIKTI
18. 2015 Atom Economical Conversion of Citronellal to Menthol over Pt-Clay nanoparticle The World Academy of Science
19. 2014 Sintesis material TiO2-SiO2 berbahan dasar abu sekam padi sebagai fotokatalis degradasi Metil Violet DPPM UII
20. 2016-2017 Slow Release Fertilizer Based on Polymer-Clay Composite Superadsorbent (Hibah Penelitian Unggulan Perguruan Tinggi, KEMENRISTEK-DIKT
21. 2016 Synthesis of TiO2-SiO2 Aerogel Using Biogenic Silica from Bamboo Leaves and Its Application for Batik Wastewater Treatment ITSF
| 1. Synthesis of TiO 2 Embedded in Al 2 O 3 -Saponite. Registration number P00200800447 |
01 July 2019 |
Patent Number : IDP000060171. Material catalyst Ti0 2 teremban the material solid support in the form of saponit pillared oxide of aluminum (TiO 2 / Al 2 O 3 -saponit) has a character area surface specific that high and dispersed homogeneously to generate the increase in energy- gap band of Ti0 2 . The method of dispersion includes the use of Al 2 O3-saponit as a material carrier , titanium isopropoxide as a source titaniurn oxide and engineering dispersion is a thing that is new in the invention of this |
| 2. Method of Making ZnO / Montmorillonite Nanoparticles with registration number : P00201100633 | 21 September 2018 | Patent Number : IDP000053596. Invention is related to a method of making nanoparticles ZnO / montmorillonite for applications photocatalysts that have had the size of a powder of 150 mesh, wide surface specifically between 9-150m 2 / g and the energy gap band between 3.2 to 3.45 eV and the content of zinc between 0, 4-15%, SiO 2 13-60%, Al 2 O 3 15-35% and Na 0.2-5.0% |
| 3. Synthesis efficiency improvement through cross-aldol condensation reaction using NaOH / ZrO 2 -Montmorillonite as a cooperative catalyst . Registration number : P00201201028 | November 13, 2018 | Patent Number : IDP000054583. The invention relates to the improvement of the efficiency of the synthesis of benzalasetone derivatives through the cross aldol condensation reaction using a new cooperative catalyst NaOH / ZrO 2- montmorillonite. The process of synthesis to increase the efficiency of the use of 0.1 g of ZrO 2 -montmorilonit each 10 ml of solution . The process of synthesis of analog benzalaseton are carried out by way of condensation aldol cross between acetone and a derivative of benzaldehyde at a temperature of 10 0 C. The purification is done by means of recrystallization with solvent ethanol-distilled water . The use of NaOH / ZrO 2- montmorillonite cooperative catalyst has been shown to increase the efficiency of the cross aldol condensation reaction . |
| 4. Method of Making Ceramic Composites for Disinfection Applications of Drinking Water from Kaolinite-Haloysite Modified by TiO 2 and ZnO . Registration Number : P00201508279 | 02 May 2019 | Patent Number : IDP000058275. Invention relates to a method of making composite ceramic antibacterial in the form of a composite based on TiO 2 , ZnO , clay kaolinite and haloysit colored white to white -gray that has a character area surface specific that high (70-300m 2 / g, is able to perform the activity of anti- bacterial in contact and photocatalytic . the new in the preparation of materials of this is the use of a combination of clay nature and composition of the constituent ceramic that is able to carry out functions in the contact-photocatalyst for the disinfection of water drinking as a matter of making ceramic antibacterial . |
Click here to chat with us!
